Key Takeaways:
- Innovative Glaucoma Implant: Ciliatech’s CID receives favorable opinions for treating both open and narrow-angle forms of glaucoma, offering new hope for patients.
- Clinical Observations: Independent observations by leading ophthalmologists highlight CID’s substantial intraocular pressure (IOP) lowering effect and safety profile.
- Global Impact: Ciliatech collaborates with global experts and regulatory authorities, aiming for commercial launches in key markets to address the needs of millions affected by glaucoma.
Subtitle: Transforming Glaucoma Treatment with Cutting-Edge Surgical Implants.
A Paradigm Shift in Glaucoma Treatment
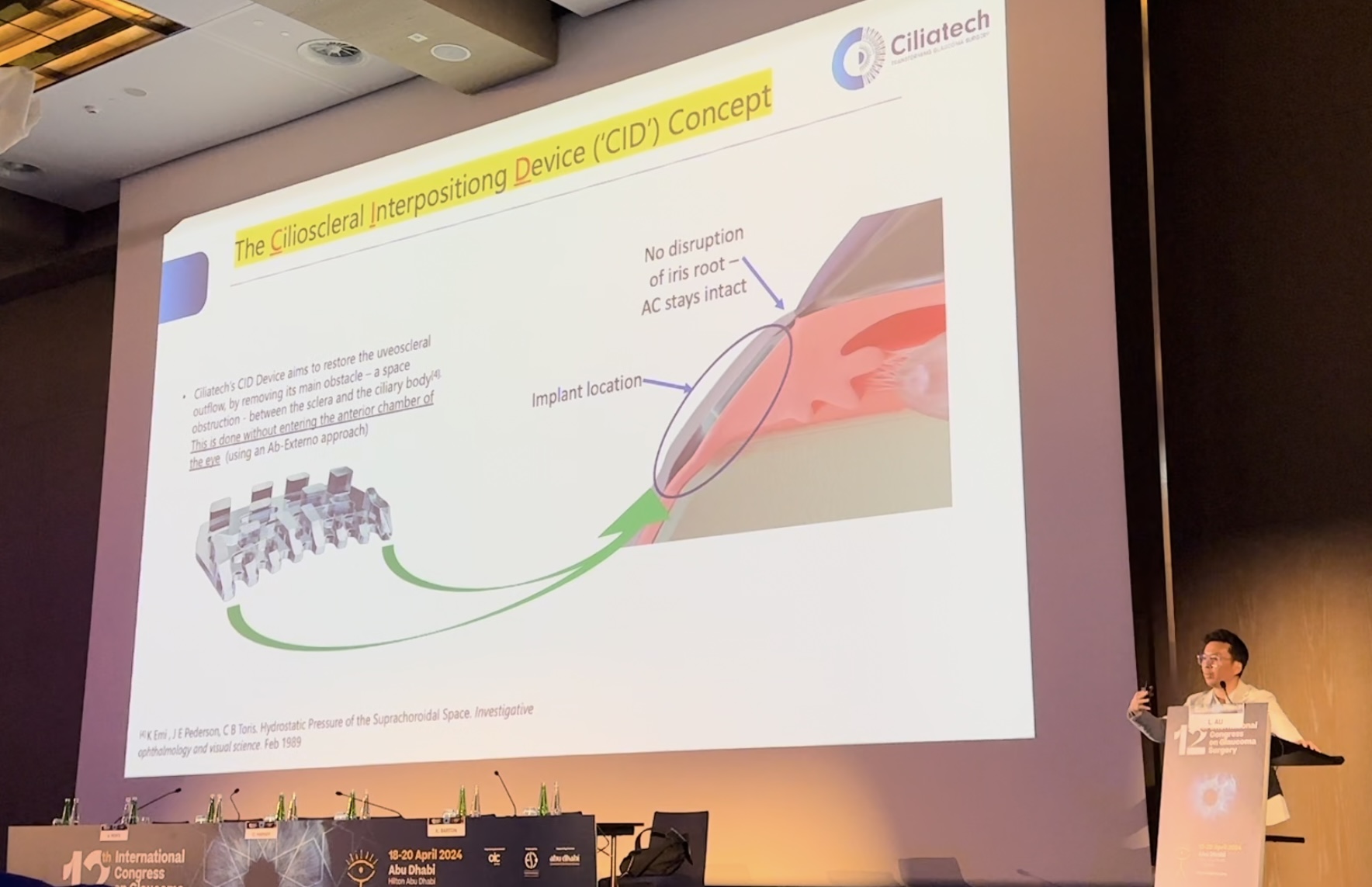
In a groundbreaking development, Ciliatech’s glaucoma surgical implant CID (Cilioscleral Interposition Device) receives favorable opinions for its efficacy in treating both open and narrow-angle forms of glaucoma, offering new hope for patients globally.
Pioneering Glaucoma Treatment
Ciliatech, an innovative medtech company specializing in glaucoma treatment, announces positive clinical observations of its CID implant during the International Congress of Glaucoma Surgery (ICGS) 2024 in Abu Dhabi. The implant, hailed as a significant advancement, showcases promising outcomes in reducing intraocular pressure (IOP) and ensuring patient safety.
Independent Validation of CID’s Efficacy
Dr. Leon Au, a distinguished consultant ophthalmologist, presented the first independent observations of CID’s performance during ICGS 2024. His remarks underscore CID’s substantial IOP-lowering effect and its potential as a game-changer in glaucoma treatment, particularly in angle closure glaucoma.
Unveiling Clinical Excellence
The clinical poster presented by Dr. Lilit Voskanyan further reinforces CID’s clinical excellence. The poster highlights significant reductions in IOP, impressive safety profiles, and the device’s efficacy in treating both primary open-angle glaucoma (POAG) and primary narrow-angle glaucoma (PNAG), offering a ray of hope to millions of patients worldwide.
Collaborative Efforts for Global Impact
Ciliatech’s collaboration with renowned ophthalmologists, surgeons, and regulatory authorities signifies a concerted effort to bring CID to global markets. The innovative implant, coupled with a robust clinical strategy and regulatory approvals, is poised to revolutionize glaucoma treatment and improve patient outcomes.
A Promising Future for Glaucoma Patients
The favorable opinions and clinical observations presented at ICGS 2024 herald a promising future for glaucoma patients. CID’s unique surgical technique and exceptional results pave the way for enhanced treatment options, ensuring better quality of life for individuals battling glaucoma.
Advancing Surgical Techniques and Technologies
CID’s innovative design and surgical implantation technique, developed by experts at Ciliatech, mark a significant advancement in glaucoma surgery. The implant’s external placement into the supraciliary space without entering the eye’s anterior chamber sets a new standard in surgical precision and patient safety.
Ciliatech’s strategic focus on regulatory approvals and commercial launches in key global markets reflects its commitment to making CID accessible to patients worldwide. The company’s relentless pursuit of scientific excellence and medical advancements reinforces its position as a leader in glaucoma treatment innovation.
Empowering Patients and Healthcare Professionals
CID’s potential to transform glaucoma treatment not only empowers patients with effective solutions but also equips healthcare professionals with advanced tools and techniques. As CID progresses through clinical trials and regulatory pathways, it heralds a new era of hope and progress in the fight against glaucoma.
Conclusion: A New Dawn in Glaucoma Care
Ciliatech’s CID implant, backed by clinical excellence and global collaborations, promises to redefine glaucoma treatment standards. With a vision to enhance patient outcomes and address unmet medical needs, CID represents a significant leap forward in the quest for innovative and effective glaucoma therapies.
Ciliatech’s CID implant emerges as a beacon of hope for millions affected by glaucoma, offering a transformative solution backed by clinical expertise and technological innovation. As the company navigates regulatory pathways and expands its global presence, CID’s potential to revolutionize glaucoma care remains unparalleled.
Sign up to our newsletter & get the most important monthly insights from around the world.
Ready to Amplify Your Brand with Business Today?
Discover the power of sponsored articles and partnerships to reach decision-makers, professionals, and a dynamic audience. Learn more about our advertising opportunities and connect with us today!
Click here to explore our Promotion & Sponsored Articles page.
Are you looking to make an impact? Contact us at pitch@businesstoday.news to get started!